Each model would apply to a defined set of single-source drugs and sole-source biological products that meet specified eligibility criteria, including spending thresholds, and would only apply to units dispensed or administered to beneficiaries selected for participation in the models. CMS proposes to test each model in geographic areas comprising approximately 25% of Medicare Part B fee-for-service (FFS) or Medicare Part D beneficiaries, respectively.
CMS states that it is relying on its authority under Section 1115A of the Social Security Act (SSA) to implement the GLOBE and GUARD Models. Section 1115A authorizes the CMS Center for Medicare and Medicaid Innovation (CMMI) to develop and test innovative payment models to reduce program expenditures while preserving or enhancing the quality of care, subject to certain criteria. The statute further permits CMS to waive various requirements under the SSA as necessary to test such models, and CMS proposes to rely on that authority to waive various provisions under the Part B and Part D inflation rebate programs to implement the models.
The GLOBE and GUARD Models are a continuation of the Trump Administration’s broader efforts to pursue MFN-based policies to lower U.S. prescription drug prices. For background on the Administration's drug pricing measures, see our November alert on the GENErating cost Reductions fOr U.S. Medicaid Model” (GENEROUS Model), our May alert on the MFN Executive Order (EO), and our April alert on the Administration's EO addressing drug pricing initiatives more generally. These proposals also follow prior efforts to implement MFN-based pricing under the Medicare program during President Trump’s first term, as discussed in our previous alert. That prior proposal was never implemented, however, after two federal courts blocked implementation: first, the United States District Court for the District of Maryland issued a temporary restraining order in Association of Community Cancer Centers v. Azar, and, second, the United States District Court for the Northern District of California issued a preliminary injunction in California Life Sciences Association v. CMS. The model was later rescinded by the Biden administration.
For the GLOBE Model, the Federal Register notice for the proposed rule is available here, CMS's press release here, and the model webpage here. For the GUARD Model, the Federal Register notice for the proposed rule is available here, CMS's press release here, and the model webpage here.
Medicare Part B GLOBE Model
The GLOBE Model would have a five-year performance period, running from October 1, 2026, through September 30, 2031, with rebate invoicing, payment, and reconciliation continuing until September 2033, for a seven-year model period overall. CMS proposes to evaluate the model’s impact in accordance with the CMMI statutory evaluation framework, including by assessing changes in Medicare spending for beneficiaries residing in model geographic areas, as well as effects on quality of care, access to drugs, and utilization patterns.
Which manufacturers would be required to participate in the GLOBE Model?
As proposed, model participation would be mandatory for all manufacturers of Part B rebatable drugs that are designated as GLOBE Model drugs, with GLOBE Model rebates due when furnished to a GLOBE Model beneficiary. CMS clarifies that a model drug may have multiple manufacturers reporting average sales price (ASP) to CMS, including when there is a manufacturer involved in the repackaging or relabeling or when more than one manufacturer markets the drug within the U.S. In those cases, all manufacturers of the model drug would be required to participate. Consistent with the definition for the Part B inflation rebate program, CMS proposes to define “manufacturer” by cross reference to the definition in the Medicaid Drug Rebate Program (MDRP) statute (42 U.S.C. § 1396r-8(k)(5)), which identifies the manufacturer of a drug by its National Drug Code (NDC).
CMS also states the following regarding manufacturers that are involved in other CMMI models: “If certain manufacturers were excluded [from the GLOBE Model] due to interactions with other CMS Innovation Center models or for any other reason, the impacts from this proposed demonstration could be significantly less than described in this analysis.”
CMS is seeking comments on factors that could drive potential exemptions, such as manufacturer size, and whether voluntary participation (as opposed to mandatory) would be sufficient for a robust model test.
Which drugs are proposed to be covered by the GLOBE Model?
Under the proposed GLOBE Model, eligible model drugs would be drugs and biologicals that satisfy all of the following criteria:
- Must qualify as a “Part B rebatable drug”: Under existing regulations, such term is defined to mean single source drugs or biological products for which payment is made under Part B, including biosimilar biological products but excluding the following (which thus are also not model drugs):
- Qualifying biosimilar biological products, defined to mean biosimilars that have an ASP not more than that of the reference biological product during a particular calendar quarter during a five-year period beginning October 1, 2022, or, for a biosimilar approved after October 1, 2022, and before December 31, 2027, the five-year period beginning with the first quarter in which a payment was made for the product.
- Products with historically excepted grouped Healthcare Common Procedure Coding System (HCPCS) codes (e.g., within the same billing and payment code as of October 1, 2003, and which are treated as multiple source drugs).
- Products billed under a not otherwise classified (NOC) code.
- Radiopharmaceutical drugs and biological products.
- Skin substitutes.
- Certain vaccines and monoclonal antibodies.
- Generic drugs (approved under Abbreviated New Drug Applications (ANDAs)).
- Drugs and biological products with average total allowed charges below the applicable threshold.
CMS is seeking comments on whether to exclude cell and gene therapies (CGTs) and plasma-derived products from the GLOBE Model.
- Must fall within at least one of the following USP therapeutic categories: Antigout agents, antineoplastics, blood products and modifiers, central nervous system agents, immunological agents, metabolic bone disease agents, or ophthalmic agents as specified in the United States Pharmacopeia Drug Classification (USP DC) published for 2025.
- Must qualify as a single source drug or sole source biological product:
- A “single source drug” would have the same meaning as defined in the ASP statute, 42 U.S.C. §1395w–3a(c)(6)(D), which means a drug that is not a multiple source drug (i.e., a drug that does not share a HCPCS code with another drug, among other requirements) and which is produced or distributed under a new drug application (NDA) approved by the Food and Drug Administration (FDA), including a drug product marketed by any cross-licensed producers or distributors operating under the NDA.
- CMS proposes to define a “sole source biological product” as a biological product licensed by the FDA under a biologics license application (BLA) and that, at time of evaluating for inclusion into the model for each applicable ASP calendar quarter, is not the reference biological product for a biosimilar licensed by the FDA in a BLA. The biosimilar must be recognized in the FDA’s Purple Book and identified as sold or marketed in FDA’s NDC Directory. CMS states in the preamble language that biosimilar biological products would be excluded from the GLOBE Model. However, that exclusion is not expressly incorporated in the proposed regulatory language.
- Must satisfy the spending threshold: Drugs must be included in a HCPCS Level II code with Medicare Part B FFS spending greater than $100 million over a 12-month period ending 6 months prior to the start of the applicable calendar quarter.
- A model drug would continue to be included in the proposed GLOBE Model once it meets this criterion for one applicable calendar quarter, even if Medicare Part B FFS spending falls below $100 million over a later 12-month period.
- Must not be in one or more of the below-described categories (which are exempted from the GLOBE Model):
- A Part B rebatable drug for which CMS has not yet established a specified amount for the first applicable calendar quarter. “This proposal would ensure that the GLOBE Model and the Medicare Part B Drug Inflation Rebate Program would treat a subsequently approved drug (that is, a drug first approved or licensed by the FDA after December 1, 2020) in a similar manner. In other words, until a specified amount is established by CMS for a subsequently approved Part B rebatable drug, that drug would not be considered for the GLOBE Model.”
- A Part B rebatable drug for which a maximum fair price (MFP) under the Medicare Drug Price Negotiation Program (DPNP) is in effect.
- A drug or biological product that is no longer a Part B rebatable drug is removed for the applicable calendar quarter in which it is no longer a Part B rebatable drug.
Once a drug or biological product has been identified as a model drug, it would remain in the GLOBE Model unless the drug or biological product becomes multiple-source (i.e., is no longer a single source drug or sole source biological product) or meets the exclusions noted above. CMS proposes to maintain a GLOBE Model Drug List that will be published on the model webpage and updated quarterly.
Which beneficiaries are proposed to be eligible?
CMS would enroll a select cohort of Medicare Part B beneficiaries, identified based on coverage and geographic criteria, which would comprise approximately 25% of the Medicare FFS population. To qualify, a beneficiary must be enrolled in Medicare Part B, have Medicare Part B FFS as his/her primary payer, and have an address of record within the GLOBE Model geographic areas selected for inclusion by CMS.
The geographic areas would be identified by ZIP code, and CMS would provide a table on the GLOBE Model website no later than 30 days before the start of the model that identifies the applicable ZIP codes. CMS would also add eligible beneficiaries to a GLOBE Model Eligible Beneficiary List and update the Medicare claims processing systems to reflect beneficiary eligibility. Once identified by CMS as a model eligible beneficiary, the beneficiary would remain in the model unless the beneficiary becomes ineligible (e.g., by no longer having Medicare Part B FFS as the primary payer). A beneficiary would remain included in the model even if the beneficiary moves to an address that is no longer in the model’s identified geographic areas.
How is the GLOBE Model rebate proposed to be calculated?
Under the GLOBE Model, manufacturers would owe an incremental GLOBE Model rebate amount when the rebate calculated using the model’s MFN-based rebate formula exceeds that under the existing statutory inflation rebate calculation. The details of this calculation are as follows:
- Incremental per unit GLOBE Model rebate amount. CMS proposes to calculate the incremental per unit GLOBE Model rebate amount by subtracting the per unit Part B inflation rebate amount from a per unit GLOBE Model rebate amount.
Incremental per unit GLOBE Model rebate amount = [(the specified amount for purposes of the Part B inflation rebate program (generally 106 percent of the lesser of the ASP or Wholesale Acquisition Cost (WAC) for an applicable quarter or rebate quarter) – per unit GLOBE Model benchmark amount) – (the same specified amount – per unit Part B inflation-adjusted payment amount)]
For example, if the specified amount is $106, the GLOBE Model benchmark amount is $70, and the inflation-adjusted payment amount is $80, the difference between the two rebate amounts (the Global Model rebate amount) would be $10 ($36 ($106-$70) - $26 ($106-$80)).
If the per unit GLOBE Model rebate amount calculates as less than $0, then CMS will set the per unit GLOBE Model rebate amount as $0.
- GLOBE Model billing units. CMS proposes to identify the GLOBE Model billing units as the billing units for an applicable quarter for purposes of the Part B inflation rebate calculation “where, on the date of service, the Medicare beneficiary was identified by CMS as a GLOBE Model eligible beneficiary and for which Medicare Part B FFS made separate payment.”
- Apportionment. Where the NDCs of more than one manufacturer are assigned to a particular billing and payment code for a GLOBE Model drug, CMS proposes to use the same methodology to apportion the rebate across manufacturers as used for Part B inflation rebates (i.e., by volume of billing units reported as sold for an NDC assigned to that billing and payment code).
- Adjustments for shortage and for severe supply chain disruption. For GLOBE Model drugs in shortage and for “GLOBE Model biosimilar biological products when there is a severe supply chain disruption,” the incremental GLOBE Model rebate amount would be reduced proportionally to the rebate reduction under the standard Part B inflation rebate program (assuming such products are included in the final GLOBE Model). Further, although CMS does not state so explicitly, to the extent that there are GLOBE Model drugs that are plasma-derived products, the shortage adjustment should also include an increased reduction, consistent with the reduction for such products under the Part B inflation rebate program. These adjustments may be time limited, again consistent with the Part B inflation rebate program.
What are the proposed benchmarks and how are they proposed to be identified?
The per unit GLOBE Model benchmark is proposed to be the greater of (1) a Method I GLOBE Model benchmark identified and calculated by CMS or (2) a Method II GLOBE Model benchmark calculated based on data reported voluntarily by manufacturers. The ultimate formula used is as follows and is described further below:
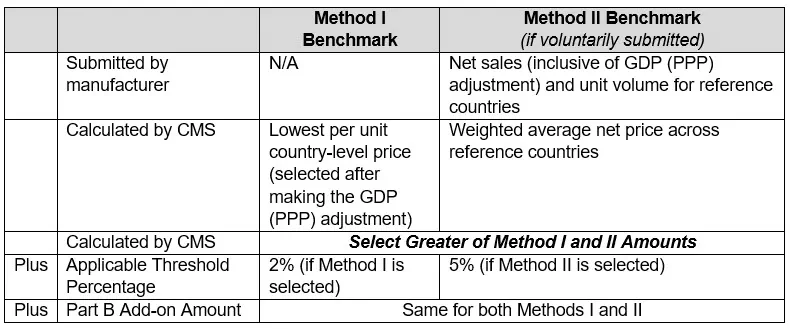
- Method I GLOBE Model benchmark. Under Method I, “CMS [proposes to] calculate[] the per unit country-level price for the international drug by country for each country . . . for which international drug pricing information is available” for the countries included in the GLOBE Model (noted below) and select the lowest per unit country-level price to use as the Method I GLOBE Model benchmark, subject to the adjustments noted below. The identification and calculation of the Method I GLOBE Model benchmark would be made as follows:
- Data and identification of international analogs: CMS would rely on data sources identified according to whether they rely on certain standardized criteria, including that data identifies relevant international analogs to the GLOBE Model drug by reference to dosage form and route of administration, strength, concentration, and units and that the data contains pricing, volume, or sales data.
- Data would be that “made available by private companies . . .” as opposed to public data sources. Examples cited by CMS include IQVIA’s MIDAS and the Global Data Pharmaceutical Prices (POLI) dataset.
- CMS would prioritize data sources that have data from the most reference countries in the model and, if available, that incorporates discounts, rebates, or other price concessions into its drug pricing information.
- CMS would exclude “pricing information at the dosage form and strength level for a country that falls below 5 percent of the average price in the U.S.”
- In contrast to the GUARD Model, where CMS suggests it may use misaligned product data to identify international analogs in certain cases, as noted below, CMS does not expressly address how it will identify the Method I GLOBE Model benchmark where there are no international analogs that align with GLOBE Model drugs based on the above criteria. Instead, CMS focuses on the process for identifying appropriate international data sources, suggesting it believes it can find a data source with an appropriate international analog.
- Benchmark price quarter: The Method I benchmark would be calculated for a single fixed quarter for each GLOBE Model drug that is “two calendar quarters prior to the first applicable calendar quarter to which the total GLOBE Model rebate amount would apply,” meaning:
- The benchmark quarter for Method I would be identified “for the first applicable calendar quarter for which the drug is a GLOBE Model drug and this benchmark [would] remain[] in place for each applicable calendar quarter thereafter until the end of the model performance period.”
- This would align with the two-quarter lag for purposes of calculating ASP-based payment limits.
- However, this single fixed quarter would not necessarily align with the Part B inflation rebate benchmark quarter, which is defined by statute, in general, as 3Q 2021 for drugs that are first or approved or licensed on or before December 1, 2020, and the third full calendar quarter after the drug is first marketed for drugs approved or licensed after December 1, 2020.
- Method II GLOBE Model benchmark. Under Method II, CMS would rely on international drug net pricing data submitted voluntarily by manufacturers for an applicable ASP calendar quarter (i.e., the quarter that is two quarters before the applicable quarter for which a rebate might be due) to calculate a volume-weighted average net price at the HCPCS Level II code billing unit level across reference countries represented. If a manufacturer does not submit international drug net pricing data, then the Method I GLOBE Model benchmark is the GLOBE Model benchmark for the applicable calendar quarter. Information reported by manufacturers for the Method II GLOBE Model benchmark is subject to the following requirements for each performance year:
- Submission requirements. Each submission would be required to include certain basic data elements. Manufacturers would be expected to submit net pricing data through one of two approaches (i.e., the “streamlined” or “limited” options) that differ in “the level of aggregation allowed for the submission of gross sales, net sales, and sales volume that was used by the manufacturer to calculate the across country volume-weighted average GDP purchasing power parity (PPP)-adjusted net pricing per HCPCS billing unit.” Both contain the same categories of data, and both would include adjustments for GDP (PPP). Manufacturers would select one submission option for all applicable international analogs to a GLOBE Model drug. Manufacturers can choose whether to make these submissions for all or only a subset of their GLOBE Model drugs.
- Benchmark price quarter: Unlike the Model I benchmark price quarter, the Model II benchmark price quarter would not be fixed but rather would float and equal the applicable ASP calendar quarter, i.e., the quarter that is two quarters before the applicable calendar quarter for which a rebate would be due.
- Data submission timing. Each submission would be due no later than 30 days after the end of the applicable ASP calendar quarter.
- Corrections and restatements. CMS proposes to allow corrections and restatements of data within 30 calendar days of the submission deadline or 5 business days of a request by CMS.
- CMS verification. CMS would “conduct a verification review to determine whether the submission meets the submission requirements.”
- Confidentiality. CMS proposes to “maintain[] the confidentiality of information submitted . . . to the extent permitted by law and in accordance with applicable privacy and security requirements.” Further, CMS proposes that it would not disclose manufacturer-submitted international drug net pricing information in a form which discloses the identity of a specific manufacturer except as CMS deems necessary to carry out the GLOBE Model calculations.
- Data agreement. A manufacturer that elects to submit international pricing information for a GLOBE Model drug must enter into a data agreement effective prior to the first submission. The agreement would remain in effect for the duration of the GLOBE Model unless terminated by either the manufacturer or CMS. CMS has not yet released the data agreement.
- Reference countries. CMS proposes criteria for identifying the set of reference countries for purposes of determining the two GLOBE Model benchmarks as “non-U.S. Organization for Economic Co-operation and Development members . . . as of October 1, 2025 with: (1) a real GDP per capita that is at least 60 percent of the U.S. real GDP per capita, as estimated and available in the Central Intelligence Agency (CIA) World Factbook; and (2) an annual real GDP that is at least $400 billion (as measured in U.S. dollars) as estimated and available in the CIA World Factbook, as determined by CMS.” If the criteria are finalized as proposed, the following set of 19 reference countries would be used: Australia, Austria, Belgium, Canada, Czechia, Denmark, France, Germany, Ireland, Israel, Italy, Japan, Netherlands, Norway, South Korea, Spain, Sweden, Switzerland, and the United Kingdom.
- GDP (PPP) adjustment. CMS proposes that data used to calculate both GLOBE Model benchmarks would be adjusted for purchasing power parity (PPP). To calculate the GDP (PPP) adjuster, CMS would “divide the U.S. real GDP per capita by the [relevant] country’s real GDP per capita and round the result to the third decimal place.” CMS would “publish a supplemental document on the GLOBE Model website with details on which GDP (PPP) adjuster would be used for each applicable ASP calendar quarter.” For the Method I GLOBE Model benchmark, the GDP (PPP) adjustment is made to each reference country’s pricing information before selecting the lowest amount as the Method I GLOBE Model benchmark. For the Method II GLOBE Model benchmark, manufacturers would make the GDP (PPP) adjustment as part of their submissions.
- Benchmark adjustments. CMS would make additional adjustments to the GLOBE Model benchmarks by applying:
- An “applicable threshold percentage” increase, which is “a modest increase to account for potential differences between the U.S. market and markets in the reference countries that may remain after allowing for economic and purchasing power differences.” For the Method I GLOBE Model benchmark, CMS proposes an “applicable threshold increase” of “a de minimus amount of up to 2 percent,” given that this benchmark “does not likely reflect the full range of confidential discounts and net pricing” and “because drug pricing information that is made available to existing data sources becomes accessible to drug purchasers, the variation among such prices for a given drug would be expected to lessen over time.” For the Method II benchmark, CMS proposes to use a higher applicable threshold percentage of 5 percent, “[t]o account for the likelihood of larger price variations across countries that may occur in actual transaction prices abroad.”
- An “add-on percentage amount,” which would equal the dollar value of the add-on percentage included in the Medicare Part B drug payment limit for the GLOBE Model drug for the applicable calendar quarter. This amount would generally be 6 percent for drugs reimbursed based on ASP.
How would beneficiary coinsurance be calculated?
Under the Part B inflation rebate program, CMS calculates a reduced beneficiary coinsurance amount where the specified amount (i.e., 106 percent of the lesser of ASP or WAC for an applicable quarter) exceeds the inflation-adjusted payment amount. This reduction is calculated as 20 percent of the inflation-adjusted payment amount instead of 20 percent of the payment limit for the quarter. The GLOBE Model would test an alternative calculation for beneficiary coinsurance where the payment limit exceeds the GLOBE Model benchmark for a GLOBE Model drug, proposed to be calculated as the lower of 20 percent of the per unit GLOBE Model benchmark amount instead (assuming it is lower than the inflation-adjusted payment amount). When the GLOBE Model reduced beneficiary coinsurance applies, “the provider or supplier would reduce the amount of coinsurance charged to the beneficiary and the portion of the Medicare Part B allowed amount that would be payable by Medicare Part B would be adjusted upwards.”
What is the proposed rebate invoicing/suggestion of error process?
CMS proposes two alternative approaches for invoicing manufacturers for GLOBE Model rebates: a “combined” model and an “incremental” model. Both approaches would rely on the same underlying GLOBE Model rebate calculation and the same per unit GLOBE Model rebate amount. What differs between the two approaches is how and when CMS would invoice manufacturers and administer the Suggestion of Error and reconciliation processes noted below. CMS intends to adopt one of the below approaches, or a “similar approach” after considering comments.
- Combined Model: Under this approach, CMS would delay Medicare Part B inflation rebate program Preliminary Rebate Reports by up to two months and provide manufacturers with a single, combined report for both the Medicare Part B inflation rebate program and the GLOBE Model. The process for the rebate report under the combined approach would align with the Medicare Part B inflation rebate program with minor modifications. While CMS proposes to issue the Preliminary Rebate Reports two months later, the regular cadence for rebate reports and reconciliations would remain unchanged. Manufacturers would use the existing Part B Suggestion of Error process to raise concerns about mathematical errors in preliminary rebate calculations.
- Incremental Model: Under this approach, CMS would invoice the GLOBE Model rebate amounts to manufacturers using a process that would be separate from, but harmonized with, the Medicare Part B inflation rebate program.
- CMS would issue the GLOBE Model Preliminary Rebate Report at least one month before issuing the GLOBE Model Rebate Report.
- Manufacturers would be required to submit a Suggestion of Error within 10 calendar days of receiving the Preliminary Rebate Report.
- CMS would issue the GLOBE Model Rebate Report no later than eight months after the end of each applicable calendar quarter.
- CMS would also adopt a reconciliation approach similar to that under the Medicare Part B inflation rebate program.
Are there proposed appeals rights?
Under the proposed rule, manufacturers would have no appeal rights with respect to CMS’s selection of model drugs, beneficiaries, or geographic areas, the total GLOBE Model rebate amount, or the calculation and application of the GLOBE Model beneficiary coinsurance percentage. CMS also proposes that the limitations on administrative or judicial review under the Part B inflation rebate program would apply to the GLOBE Model. Section 1115A(d)(2) of the SSA, the statute governing the authority of CMMI, also provides limitations on administrative and judicial review of aspects relating to CMMI models, including (but not limited to) the selection of models, organizations, sites, and participants involved in the models and the elements, parameters, scope, and duration of such models for testing.
Manufacturers would, however, have the right to appeal any CMPs imposed by CMS under the model, including determinations that the incremental GLOBE Model rebate amount due was not paid by the applicable payment deadline and the calculation of CMS amount. Appeals would proceed under CMS’s existing CMP appeal procedures, including the right to a hearing following the exhaustion of administrative remedies.
Are there proposed penalties?
Under the proposed rule, a manufacturer that fails to timely pay the incremental GLOBE Model rebate amount would be subject to a civil monetary penalty (CMP) equal to at least 125% of the incremental rebate amount due for the drug. A manufacturer that knowingly fails to comply with the GLOBE Model requirements, including the provisions in the data agreement, would also be subject to a CMP. A CMP would be in addition to any unpaid incremental rebate amount, and payment would be due within 60 days of the notice of imposition. Additionally, CMS proposes that if one or more grounds for enforcement action are met, it may take additional enforcement actions, including terminating the data agreement with the manufacturer, requiring the manufacturer to provide additional requested information to CMS or its designees, or subjecting the manufacturer to additional monitoring, auditing, or both. CMS would also have the authority to impose CMPs or terminate a manufacturer’s data agreement if the manufacturer submits false data or is subject to investigation or action by the U.S. Department of Health and Human Services (HHS) or the Department of Justice (DOJ) due to an allegation of fraud or significant misconduct.
Are there statutory provisions that would be waived?
For purposes of executing the above proposed GLOBE Model under Section 1115A of the SSA, CMS proposes to waive, to the extent necessary:
- The provisions of the Part B inflation rebate program calculation under Section 1847A(i)(3) of the SSA (42 U.S.C. §1395w–3a(i)(3)) and implementing regulations,
- The Part B inflation rebate program invoice timing requirements under Section 1847A(i)(1) of the SSA and implementing regulations, and
- Various provisions of Section 1833 of the SSA related to beneficiary coinsurance and the Medicare portion of payment for a drug and implementing regulations.
What are the proposed government price reporting obligations?
In the preamble to the GLOBE Model proposed rule, CMS specifies that GLOBE Model rebates would be excluded from (i.e., not reduce) a manufacturer’s average manufacturer price (AMP), best price, and ASP calculations (and thus would not impact the 340B ceiling price). By statute, Part B inflation rebates made under Section 1847A(i) of the SSA are excluded from AMP and best price and sales exempt from best price are exempt from ASP. CMS states that “the GLOBE Model rebate amounts are rebates under Section 1847A(i),” meaning that CMS interprets the exclusion that applies to those rebates to carry over to the GLOBE Model rebates.
Medicare Part D GUARD Model
The GUARD Model would have a five-year performance period, running from January 1, 2027, through December 31, 2031, with rebate invoicing, payment, and reconciliation continuing until December 31, 2033, for a seven-year model period overall. CMS proposes to evaluate the model’s impact in accordance with the CMMI statutory evaluation framework. Evaluation would include analysis of changes in net Medicare Part D spending and total Medicare spending for beneficiaries residing in model geographic areas, out-of-pocket costs, utilization patterns, and quality of care.
Which manufacturers would be required to participate in the GUARD Model?
As proposed, participation in the GUARD Model would be mandatory for all manufacturers of Part D rebatable drugs that are designated as GUARD Model drugs, with GUARD Model rebates due when furnished to a GUARD Model beneficiary. CMS did not propose any exceptions for eligible manufacturers. Consistent with the definition for the Part D inflation rebate program, CMS proposes to define “manufacturer” by cross reference to the definition in the MDRP statute (42 U.S.C. § 1396r-8(k)(5)), which identifies the manufacturer of a drug by its NDC.
As in the GLOBE Model, CMS also states the following, regarding manufacturers that are involved in other CMMI models: “If certain manufacturers were excluded due to interactions with other CMS Innovation Center models or for any other reason, the impacts from this proposed demonstration could be significantly less than described in this analysis.”
CMS is seeking comments on whether voluntary participation (as opposed to mandatory) would be sufficient for a robust model test.
Which drugs are proposed to be covered by the GUARD Model?
Under the proposed GUARD Model, eligible model drugs would be drugs and biologicals that satisfy all of the following criteria:
- Must qualify as a “Part D rebatable drug”: Under existing regulations such term is defined to mean a drug that is a covered Part D drug that approved under an NDA or a biological licensed under a BLA, including biosimilar, or certain generics approved under an ANDA, but excluding the following:
- Drugs not covered under a MDRP agreement.
- Products that do not meet the definition of a “covered outpatient drug” under the MDRP (e.g., vaccines, certain diabetes supplies).
- Drugs with average total Part D costs below the applicable threshold.
A Part D rebatable drug would be subject to the exclusions discussed below in order to qualify as a GUARD Model drug.
- Must fall within at least one of the following therapeutic categories in the USP Medicare Model Guidelines: Analgesics; Anticonvulsants; Antidepressants; Antimigraine Agents; Antineoplastics; Antipsychotics; Antivirals; Bipolar Agents; Blood Glucose Regulators; Cardiovascular Agents; Central Nervous System Agents; Gastrointestinal Agents; Genetic or Enzyme or Protein Disorder: Replacement or Modifiers or Treatment; Immunological Agents; Metabolic Bone Disease Agents; Ophthalmic Agents; and Respiratory Tract/Pulmonary Agents. This list includes the current USP categories that correspond to the Medicare Protected Classes, as defined in Chapter 6 section 30.2.5 from the Medicare Prescription Drug Benefit Manual.
- This approach differs from the GLOBE Model, where CMS proposes to rely on the USP DC.
- Must qualify as a single source drug or sole source biological product:
- CMS proposes to define a “single source drug” as a drug approved by the FDA under an NDA for which there are no generics rated as therapeutically equivalent. The generic must be recognized as a therapeutic equivalent in the FDA’s Orange Book and identified as marketed in the FDA’s NDC Directory.
- CMS proposes to define a “sole source biological product” as a biological product licensed by the FDA under BLA and that is not the reference biological product for a biosimilar licensed by the FDA in a BLA. The biosimilar must be recognized in the FDA’s Purple Book and identified as sold or marketed in FDA’s NDC Directory.
- Must not be in one or more of the below-described categories (which are exempted from the GUARD Model):
- Generics and biosimilar biological products (except for qualifying authorized generics and unbranded biological products if they qualify as Part D rebatable drugs).
- A drug with application-level total gross covered prescription drug costs below the GUARD Model minimum spend threshold: $69 million for the first performance year and adjusted annually to account for inflation. Once a model drug exceeds the minimum spend threshold for a performance year during the model performance period, the model drug would no longer be subject to the exclusion for subsequent performance years.
- Unlike in the GLOBE Model in which CMS addresses the spending threshold as an eligibility factor, CMS addresses the spending threshold as an exclusion in the GUARD Model.
- A Part D rebatable drug for which an MFP under the DPNP is in effect.
Once a drug or biological product has been identified as a model drug, it would remain in the GUARD Model unless the drug or biological product becomes multiple-source (i.e., is no longer a single source drug or sole source biological product) or meets the exclusions noted above.
CMS’s approach to rebatable drugs under the GUARD Model is distinguishable from the GLOBE model in the following additional respects:
- CMS does not request comment on whether to exclude CGTs and plasma-derived products as it does for the GLOBE Model.
- CMS does not propose to maintain a GUARD Model Drug List that will be published on the model webpage as it does with the GLOBE Model.
- CMS does not propose to incorporate the following exclusions to the GUARD Model where parallel exclusions are proposed for the GLOBE Model: a Part D rebatable drug for which CMS has not yet established an Annual Manufacturer Price for the first applicable period; and a drug or biological product that is no longer a Part D rebatable drug is removed for the applicable period in which it is no longer a Part D rebatable drug.
Which beneficiaries would be included?
CMS would enroll a select cohort of Medicare Part D beneficiaries, identified based on coverage and geographic criteria, which would comprise approximately 25% of Part D beneficiaries. To qualify, a beneficiary must be a Part D enrollee whose home address as recorded in CMS’ Medicare Enrollment Database or CMS’ Medicare Beneficiary Database (MDB) System is within the set of ZIP Codes linked to ZIP Code Tabulation Areas (ZCTAs) selected for the model geographic areas. In comparison, the GLOBE Model’s beneficiary geographic eligibility criteria would be an address of record, identified directly by a ZIP code selected for inclusion by CMS.
CMS would provide a table on the GUARD Model website no later than 60 days before the start of the model that identifies the applicable geographic areas. CMS would also identify eligible GUARD Model beneficiaries no later than 30 days before the start of the model and identify subsequent eligible beneficiaries periodically thereafter. Once identified by CMS as a model eligible beneficiary, the beneficiary would remain in the model unless the beneficiary becomes ineligible (e.g., by no longer being enrolled in Part D). A beneficiary would remain included in the model even if the beneficiary moves to an address that is no longer in the model’s identified geographic areas.
How is the GUARD Model rebate proposed to be calculated?
Under the GUARD Model, manufacturers would owe an incremental GUARD Model rebate amount when the rebate calculated using the GUARD Model’s MFN-based rebate formula exceeds the amount under the existing Part D inflationary rebate calculation for an NDC-9, if any. The details of this calculation are as follows:
- Incremental per unit GUARD Model rebate amount. CMS proposes to calculate the incremental per unit GUARD Model rebate amount per NDC-9 by subtracting the per unit “Part D inflation rebate amount” (defined below) for that NDC-9 from a per unit GUARD Model rebate amount for that NDC-9, which is calculated as the per unit “Medicare net price” (defined below) less the per unit applicable international benchmark (also described below).
Incremental per unit GUARD Model rebate amount = (per unit Medicare net price – per unit applicable international benchmark) – per unit Part D inflation rebate amount (as adjusted to reflect WAC and National Council for Prescription Drug Programs (NCPDP) units).
For example, if the per unit Medicare net price is $196 and the per unit applicable international benchmark is $106, then the per unit GUARD Model rebate amount would be $90. The incremental per unit GUARD Model rebate amount would then be the difference between $90 and a per unit Part D inflation rebate amount of $80 or $10.
Where this amount calculates to $0 or less, the incremental per unit GUARD Model rebate amount is set to $0.
- Per unit Medicare net price. In general, CMS proposes to calculate the per unit Medicare net price at the NDC-9 level by first calculating the performance year aggregate gross price based on WAC across all NDC-11s and Prescription Drug Event (PDE) records of a drug, less (1) manufacturer rebates derived from detailed Direct and Indirect Remuneration (DIR) reports from Part D plan sponsors, and (2) discount amounts provided by manufacturers via the Manufacturer Discount Program, all of which is then divided by the sum of the total quantity dispensed across all PDE records during the performance year. CMS seeks feedback on whether a metric other than WAC should be used to calculate the Medicare net price.
- Per unit Part D inflation rebate amount. The per unit Part D inflation rebate amount is calculated by statute based on AMP and AMP units. As a result, CMS proposes to convert this amount using WAC and NCPDP units to match the Medicare net price formula, before calculating the incremental per unit GUARD Model rebate amount.
- GUARD Model units. CMS proposes to define the GUARD Model billing units for a performance year as the same units used to calculate the Part D inflation rebate amount in an applicable period for purposes of that program. As a result, consistent with the Part D inflation rebate program, this amount would exclude units for which the manufacturer provides a discount under the 340B program and units associated of compounded drugs and would further exclude units for PDE records not associated with a GUARD Model beneficiary.
- Adjustments for shortage, severe supply chain disruption, and for generic drugs likely to be in shortage. For GUARD Model drugs in shortage, for generic and biosimilar biological GUARD Model drugs when there is a severe supply chain disruption, and for generic GUARD Model drugs that are likely to be in shortage, the incremental GUARD Model rebate amount would be reduced proportionally to the rebate reduction under the standard Part D inflation rebate program. Although CMS does not say so explicitly, the adjustment for GUARD Model drugs in shortage would include an increased reduction for generics and plasma-derived products to the extent included in the GUARD Model, as that reduction is applied consistent with the reduction for such products under the Part D inflation rebate program. These adjustments may be time limited, again consistent with the Part D inflation rebate program.
What are the GUARD Model proposed benchmarks and how are they proposed to be identified?
The per unit GUARD Model benchmark is proposed to be the greater of (1) the default international benchmark (also referred to as Method I); or, if available, (2) the updated international benchmark (also referred to as Method II). Notably, the GLOBE Model proposes the use of the same type of benchmark prices for Model I and II but does not label them as “default” and “updated” as the GUARD model. The ultimate formula used is as follows and is described further below:

- Default international/Method I GUARD Model benchmark. The default international/Method I GUARD Model benchmark (Method I) for each GUARD Model drug would be the lowest per unit country-level average price among the set of per unit prices, to the extent available, for the countries included in the GUARD Model, subject to the adjustments noted below. The identification and calculation of the Method I would be made as follows:
- Data and identification of international analogs: CMS would rely on data sources identified according to whether they rely on certain standardized criteria, including that data identifies relevant international analogs to the GUARD Model drug by reference to dosage form and route of administration, strength, and units, and that the data contains pricing, volume, or sales data. Concentration is not expressly addressed for the GUARD Model, although it is addressed in the GLOBE Model.
- CMS also appears to be looking to data from private companies for purposes of identifying this benchmark, given that it used “proprietary global drug pricing data sources” in preparing the proposed GUARD Model to confirm the sufficiency of data. Examples cited by CMS include IQVIA’s MIDAS and the POLI dataset, which examples were also cited for the GLOBE Model.
- CMS proposes to prioritize data sources that have data from the most reference countries in the model and solicits feedback on how to weigh data sources with fewer reference countries but that incorporate discounts, rebates, or other price concessions into its drug pricing information.
- CMS considered proposing to exclude data sources with pricing information that falls below a specific threshold of prices in the United States but ultimately decided not to.
- In the preamble to the GUARD Model proposed rule, CMS suggests that where there are “no fully aligned” international analogs to the GUARD Model drug based on these criteria, it may consider other international analogs, even where those analogs are “strength misaligned” to the GUARD Model drug. Thus, in contrast to the GLOBE Model discussed previously, CMS appears open to the possibility that it may use pricing data for an international product that does not exactly align with the characteristics of the GUARD Model drug.
- Benchmark performance year: The Method I benchmark would be calculated for a single fixed performance year based on the first performance year that a GUARD Model drug is included in the model, meaning:
- The benchmark for Method I would be identified “once during the GUARD Model performance period” for each GUARD Model drug and “would not be revised over time with more recent international pricing data.”
- This single fixed performance year with respect to each GUARD Model drug would not necessarily align with the Part D inflation rebate benchmark period, which is defined by statute, in general, as 1Q 2021 to 3Q 2021 for drugs that are first or approved or licensed on or before October 1, 2021, and the first calendar year after the drug is first marketed for drugs approved or licensed after October 1, 2021.
- The performance year under the GUARD Model, and thus the Model I benchmark period, would be calculated based on a calendar year period.
- Updated international/Method II GUARD Model benchmark. Under the updated international/Method II benchmark (Method II), CMS would rely on international net pricing data submitted voluntarily by manufacturers for an applicable performance year to calculate an across-country average net price. Each submission would be used only “for the specific corresponding performance year, and any subsequent performance year would need a separate submission.” If a manufacturer does not submit international drug net pricing data, then the GUARD Model benchmark will be based on Method I.
- Submission requirements. Each submission would be required to include certain basic data elements. Manufacturers further would be expected to submit net pricing data through one of two approaches, i.e., the “streamlined” or “limited” data options, that “differ in granularity of data submitted” but which both contain information on the gross sales, net sales, and sales volume used by the manufacturer to calculate the across-country average net price, adjusted for GDP (PPP). Manufacturers would select one submission option for all applicable international analogs to a GUARD Model drug. Manufacturers can choose whether to make these submissions for all or only a subset of their GUARD Model drugs.
- Benchmark performance year: Unlike Method I, the Method II benchmark price quarter would not be fixed but rather would float and equal the performance year for which an incremental GUARD Model rebate might be due.
- Data submission timing. Each submission would be due within 180 calendar days of the end of the performance year.
- Corrections and resubmissions. CMS proposes to allow corrections and restatements of applicable submissions within 30 days of the submission deadline or 15 calendar days of a request by CMS.
- CMS verification. CMS proposes to conduct a verification review to determine whether the submission requirements are met.
- Confidentiality. CMS proposes to “maintain confidentiality of information submitted . . . to the extent permitted by law and in accordance with applicable privacy and security requirements.” CMS, however, does not provide the same assurances as it does under the GLOBE Model that it would not disclose manufacturer-submitted international drug net pricing information in a form which discloses the identity of a specific manufacturer.
- Data agreement. Manufacturers would also be required to execute a data agreement 90 days prior to submission of updated international/Method II GUARD Model benchmark data for each performance year. This means that manufacturers would need to sign this agreement by 90 days after the end of a performance year, given that, as noted below, submission of data would be due 180 calendar days after the end of the 2027 performance year.
- Reference countries. In general, for the GUARD Model, CMS proposes to use the same criteria for identifying reference countries as for GLOBE, resulting in the same 19 reference countries.
- GDP (PPP) adjustment. Both GUARD Model benchmarks would reflect prices adjusted for purchasing power parity, using the same GDP (PPP) adjusters as under GLOBE. The GDP (PPP) adjuster is calculated by dividing the U.S. GDP (PPP) by the reference country’s GDP (PPP) and may not be less than one. For the default Method I GUARD Model benchmark, GDP (PPP) adjustment is made to each reference country’s pricing information before selecting the lowest amount as the default international/Method I GUARD Model benchmark. For the updated Method II GUARD Model benchmark, manufacturers would make the GDP (PPP) adjustment as part of their submissions.
- Benchmark adjustments. After CMS selects the greater of the default international benchmark or the updated international benchmark, CMS would apply an additional applicable adjustment factor:
- If based on the default international/Method I GUARD Model benchmark, the applicable adjustment factor is 102 percent.
- If based on the updated international/Method II GUARD Model benchmark, the applicable adjustment factor is 105 percent.
What is the proposed rebate invoicing/suggestion of error process?
CMS proposes the following timeline for invoicing manufacturers:
- CMS would issue the Preliminary GUARD Model Rebate Report at least one month before issuing the GUARD Model Rebate Report, and no later than 20 months after the end of the performance year.
- Manufacturers would be required to submit a Suggestion of Error within 10 calendar days from the date of receipt of the Preliminary Rebate Report.
- CMS would issue the GUARD Model Rebate Report no later than 22 months after the end of each performance year, and manufacturers would have 30 calendar days from that date to make payment.
- CMS would also adopt a reconciliation approach similar to that under the standard Medicare Part D inflation rebate program.
Are there proposed appeals rights?
Under the proposed rule, manufacturers would have no appeal rights with respect to CMS’s selection of model drugs, beneficiaries, or geographic areas, the total GUARD Model rebate amount, or specific data inputs or calculations related to the rebate amount. CMS also proposes that the limitations on administrative or judicial review under the Part D inflation rebate program would apply to the GUARD Model. As mentioned above, Section 1115A(d)(2) of the SSA also provides limitations on administrative and juridical review of aspects relating to CMMI models.
Manufacturers would, however, have the right to appeal CMPs imposed under the GUARD Model, including determinations that an incremental GUARD Model rebate amount due was not paid by the applicable payment deadline and the calculation of CMP amount. Appeals would proceed under existing CMP appeal procedures, including the right to a hearing following exhaustion of administrative remedies.
Are there proposed penalties?
Under the proposed rule, a manufacturer that fails to timely pay the GUARD Model rebate amount or knowingly fails to comply with GUARD Model requirements would be subject to a CMP equal to 125% of the incremental rebate amount due for the drug for a performance year. The CMP would be in addition to any unpaid rebate amount due.
Are there statutory provisions that would be waived?
For purposes of executing the above proposed GUARD Model under Section 1115A of the SSA, CMS proposes to waive, to the extent necessary:
- The provisions of the Part D inflation rebate program calculation under Section 1860D–14B(b)(1) the SSA (42 U.S.C. § 1395w-114b(b)(1)) and
- The Part D inflation rebate program invoice timing requirements under Section 1860D–14B(a)(1) the SSA.
What are the proposed government price reporting obligations?
CMS does not address the treatment of the GUARD Model rebates in AMP, best price, and ASP for purposes of government price reporting. However, Part D inflation rebates are excluded from (i.e., do not reduce) AMP and best price by statute and sales exempt from best price are exempt from ASP. CMS did not propose to waive any of these exclusions with respect to Part D inflation rebates with respect to the GUARD Model rebates. As a result, GUARD Model rebates appear to be excluded from AMP and best price and, correspondingly, ASP as well, consistent with the GLOBE Model.
Conclusion
We will continue to monitor for updates to the proposed GLOBE and GUARD Models as well as for other developments from CMS and the Administration related to MFN pricing policies. As always, it is important that you carefully review the models to identify all issues relevant to your organization. If you have any questions about what these developments may mean in practice, please contact any of the authors of this update or the Hogan Lovells lawyer with whom you regularly work.
Authored by Alice Valder Curran, Melissa Bianchi, Beth Roberts, Stuart Langbein, Beth Halpern, Maura Calsyn, Samantha Marshall, Kathleen Peterson, Mahmud Brifkani, Caroline Farrington, Sydney Fay, and Viraj Paul.

 Search
Search


 Search
Search


